RÚV
13.07.2022
Berlingske greinir frá þessu en stofnun sem sér um kvartanirnar hefur afgreitt um helming þeirra.
Alls hafa ellefu hundruð þrjátíu og átta sótt um bætur. Þar af hefur stofnunin samþykkt kröfur þrjátíu og tveggja en hafnað um fimm hundruð.
Danska ríkisútvarpið hefur eftir Karen-Inger Bast, framkvæmdastjóra stofnunarinnar, að sanna þurfi að tengsl séu á milli bólusetningar og þeirra líkamlegu kvilla sem hrjá umsækjendur.
Það hafi alla jafna ekki tekist. Þá þurfi aukaverkanirnar að vera alvarlegar og sjaldæfar.
Að sögn Bast sækja um tíu til fimmtán um í hverri viku. Það hljómi eins og mikill fjöldi en hafa beri í huga hversu margir voru bólusettir á skömmum tíma gegn kórónuveirunni. Í því samhengi sé fjöldinn ekki svo mikill.
Lágt hlutfall samþykktra umsókna sýnir að bóluefnin eru örugg, að sögn Bast. Vissulega veikist margir eftir bólusetningu en þau veikindi séu ekki endilega tengd sprautunni.
Tíu af þeim sem fengu bætur glíma við lömun í andliti eftir bólusetninguna og sex fengu blóðtappa.
SKRÁÐAR AUKAVERKANIR HJÁ LYFJASTOFNUN ÍSLANDS

Sjá https://www.lyfjastofnun.is/covid-19/aukaverkanatilkynningar-vegna-covid-19
Sundurliðun tilkynninga
Til dagsins í dag hafa 293 tilkynningar vegna gruns um alvarlega aukaverkun* borist Lyfjastofnun. Skiptast þær svo milli bóluefnanna:
Comirnaty (BioNTech/Pfizer):
149 alvarlegar tilkynningar hafa borist.
- 27 þeirra varða andlát. 18 andlát vörðuðu aldraða** einstaklinga, 15 þeirra með staðfesta undirliggjandi sjúkdóma. Sjö andlát vörðuðu eldri*** einstaklinga, fjórir þeirra með staðfesta undirliggjandi sjúkdóma. Tvö andlát varða einstaklinga á aldursbilinu 55-64 ára, þar sem annar þeirra var með staðfestan undirliggjandi sjúkdóm.
- 80 tilkynningar varða sjúkrahúsvist (þar af þrettán lífshættulegt ástand).
- 26 tilkynningar teljast klínískt mikilvægar og þar með flokkaðar sem alvarlegar****.
- Ellefu tilkynningar teljast alvarlegar, þar sem beðið er eftir viðbótar upplýsingum.
- Fimm tilkynningar varða fósturmiska.
Spikevax (Moderna):
41 alvarlegar tilkynningar hafa borist.
- Ein tilkynning varðar andlát aldraðs** einstaklings.
- 30 tilkynningar varða sjúkrahúsvist (þar af tvær lífshættulegt ástand).
- Ein tilkynning varðar lífshættulegt ástand þar sem ekki kom til sjúkrahúsvistar.
- Fjórar tilkynningar teljast klínískt mikilvægar og þar með flokkaðar sem alvarlegar****.
- Ein tilkynning telst alvarleg, þar sem beðið er eftir viðbótar upplýsingum.
- Fjórar tilkynningar varða fósturmiska.
Vaxzevria (AstraZeneca):
84 alvarlegar tilkynningar hafa borist.
- Sjö tilkynningar varða andlát; fjögur andlát varða eldri*** einstaklinga, tveir þeirra með staðfesta undirliggjandi sjúkdóma. Þrjár tilkynningar varða andlát einstaklinga á aldursbilinu 60-64 ára; Einn þeirra var með staðfestan undirliggjandi sjúkdóm.
- 60 tilkynningar varða sjúkrahúsvist (þar af 24 lífshættulegt ástand).
- 13 tilkynningar teljast klínískt mikilvægar og þar með flokkaðar sem alvarlegar****.
- Þrjár tilkynningar teljast alvarlegar, þar sem beðið er eftir viðbótar upplýsingum.
- Ein tilkynning varðar fósturmiska.
COVID-19 Vaccine Janssen:
19 alvarlegar tilkynningar hafa borist.
- Ein tilkynning varðar andlát eldri*** einstaklings
- 13 tilkynningar varða sjúkrahúsvist (þar af þrjár lífshættulegt ástand).
- Þrjár tilkynningar teljast klínískt mikilvægar og þar með flokkaðar sem alvarlegar****.
- Ein tilkynning telst alvarleg, þar sem beðið er eftir viðbótar upplýsingum.
- Ein tilkynning varðar fósturmiska.
*Alvarleg aukaverkun er aukaverkun eða óæskileg áhrif lyfs sem leiðir til dauða, lífshættulegs ástands, sjúkrahúsvistar eða lengingar á sjúkrahúsvist, veldur fötlun eða fæðingargalla hjá mönnum.
** Aldraðir einstaklingar eru hér skilgreindir 75 og eldri.
*** Eldri einstaklingar eru hér skilgreindir á aldursbilinu 65-74 ára.
****Tilkynningar sem metnar eru sem klínískt mikilvægar geta varðað ýmis einkenni, t.d. blóðtappa þar sem ekki kom til innlagnar á sjúkrahús.
Fjöldi tilkynninga vegna gruns um aukaverkanir og alvarlega aukaverkun í kjölfar bólusetningar í aldurshópnum 5-11 ára:
- Tólf tilkynningar hafa borist og þar af er engin alvarleg.
Fjöldi tilkynninga vegna gruns um aukaverkanir og alvarlega aukaverkun í kjölfar bólusetningar í aldurshópnum 12-15 ára:
- 44 tilkynningar hafa borist og þar af eru fjórar alvarlegar.
Tilkynningar um aukaverkanir í kjölfar bólusetningar með örvunarskammti
- Í ágúst 2021 bárust þrjár tilkynningar um alvarlegar aukaverkanir sem tengdust örvunarbólusetningu. Tvær þeirra vörðuðu bóluefnið Comirnaty og ein Spikevax.
- Í september 2021 barst ein tilkynning um alvarlega aukaverkun sem varðaði örvunarbólusetningu með Comirnaty.
- Í október 2021 bárust fjórar tilkynningar um alvarlegar aukaverkanir vegna örvunarbólusetningar. Tvær þeirra vörðuðu bóluefnið Comirnaty og hinar tvær Spikevax.
- Í nóvember 2021 bárust fjórar tilkynningar um alvarlegar aukaverkanir í kjölfar örvunarbólusetningar og vörðuðu þær allar bóluefnið Comirnaty.
- Í desember 2021 bárust sex tilkynningar um alvarlegar aukaverkanir vegna örvunarbólusetningar. Fjórar þeirra vörðuðu bóluefnið Comirnaty og hinar tvær Spikevax.
- Í janúar 2022 bárust fjórar tilkynningar um alvarlegar aukaverkanir sem tengdust örvunarbólusetningu. Þrjár þeirra vörðuðu bóluefnið Comirnaty og ein Spikevax.
- Í febrúar 2022 liggja tölur ekki fyrir um alvarlegar aukaverkanir sem tengdust örvunarbólusetningu.
- Tölur um alvarlegar aukaverkanir varðandi örvunarbólusetningu í mars 2022 liggja ekki fyrir.
- Tölur um alvarlegar aukaverkanir sem varða örvunarbólusetningu í apríl 2022 liggja ekki fyrir.

18 januar 2022
Upplýsingar um tilkynntar aukaverkanir á Norðurlöndum
Danmörk – upplýsingar á vef LægemiddelstyrelsenOpna
Finnland – upplýsingar á vef FIMEA Opna
Noregur – upplýsingar á vef Statens legemiddelverk Opna
Svíþjóð – upplýsingar á vef Läkemedelsverket Opna
HEIMASÍÐA DÖNSKU LYFJASTOFNUNARINNAR
Suspected side effects reported for COVID-19 vaccines
Like all medicines the COVID-19 vaccines can cause side effects. Common to all vaccines is that the side effects that could occur after vaccination must not be worse than the disease that they are meant to prevent.
All COVID-19 vaccines
Overview of suspected side effects reported for COVID-19 vaccines
Updated on 18 January 2022

The vaccine’s known side effects appear from the product information of Comirnaty (Pfizer/BioNTech).
Status update on the monitoring of side effects in Denmark
Updated on 18 January 2022.
The Danish Medicines Agency has received 33,252 reports of suspected side effects of Comirnaty (Pfizer/BioNTech), of which we have reviewed 13,688. Reports of serious suspected side effects are reviewed first.
When healthcare professionals, members of the public or their carers/relatives report suspected side effects to the Danish Medicines Agency, only a suspicion is needed to report symptoms they think are side effects of a certain medicine. In other words, a reported suspected side effect does not mean that there is an established connection between the medicine and the experienced symptoms.
The vast majority of suspected side effects reported after vaccination are mild and moderate. They usually involve known and transient side effects such as reactions at the injection site (e.g. pain and itching), tiredness, headache, muscle pain, joint pain, nausea and fever.
When a vaccine activates the immune system, many will experience flu-like symptoms. This is completely normal and a sign that the body’s immune system is reacting to the vaccine. These reactions occur with most vaccine types, and they usually pass within a couple of days.
However, if the immune system does not react, it does not mean that the vaccine is not working. People simply react differently to being vaccinated.
Table 10 below shows the ten most frequently reported suspected side effects (based on cases reviewed).
| The most frequently reported suspected side effects of Comirnaty (Pfizer/BioNTech) as of 18 January 2022 |
|---|
| Headache |
| Fever |
| Tiredness |
| Muscle pain |
| Pain at injection site |
| Nausea |
| Joint pain |
| Dizziness* |
| Chills |
| Feeling unwell |
*Dizziness is not described as a known side effect in the product information, but dizziness may occur in connection with flu-like symptoms, such as fever, chills, tiredness and a feeling of being unwell. These are known side effects of the vaccine.
The table below shows the percentage distribution by age of the reports of suspected side effects received and reviewed by the Danish Medicines Agency for Comirnaty (Pfizer/BioNTech):
| Percentage distribution of reviewed reports by age groups (years) for Comirnaty (Pfizer/BioNTech) as of 18 January 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 | 5-11 | 12-15 | 16-19 | 20-39 | 40-64 | 65-79 | 80+ | Ukendt alder | |
| 0,1% | 0,8% | 2,2 % | 3,2 % | 31,5% | 47,4 % | 8,2 % | 5,5 % | 1,1 % | |
When the Danish Medicines Agency monitors the safety of the COVID-19 vaccines – and medicines in general – we pay particular attention to reports of suspected serious and unexpected side effects. This means that we give first priority to these reports and assess them thoroughly to find out if there is a possible link to the vaccine. In this assessment, we may need to obtain additional information about the side effect.
We also monitor suspected side effects after the COVID-19 booster vaccination. Currently, the Danish Medicines Agency has no reason to suspect that the side effect profile of the booster vaccine is any different from the vaccine’s general side effect profile.
Since the vaccine rollout began in Denmark, we have monitored the below focus areas closely as part of the safety monitoring of the COVID-19 vaccines.
Deaths
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of deaths in the time after vaccination.
The Danish Medicines Agency has so far received and assessed 124 reports involving deaths in the time after vaccination. In all 124 cases, it was assessed to be less likely that the deaths were connected to the vaccine, and that the deaths were more likely to have been caused by other factors.
The reports of deaths are being closely monitored in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Blood clots
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of blood clots in the time after vaccination.
At this time, the Danish Medicines Agency does not suspect the vaccine to cause side effects in the form of blood clots. Blood clots can be explained by several other factors such as the person’s health conditions, including other diseases and medication taken. In addition, the needle prick itself from the vaccine jab may – even when administered correctly – in rare cases cause blood clots. So, blood clots can occur in the time after vaccination without being related to the vaccine.
The reports of blood clots are monitored closely in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Children
The Danish Medicines Agency has particular focus on the monitoring of suspected side effects in the vaccination groups of children aged 5 to 11 years and children aged 12 to 15 years. Based on the available data, the general side effect profile for these age groups is comparable to the observations made for the rest of the population, which primarily involve known and transient side effects.
Pregnancy and breast-feeding
The Danish Medicines Agency has particular focus on the monitoring of suspected side effects in pregnant women and breast-feeding women. The Danish Medicines Agency’s monitoring has given no reason to suspect the side effect profile of this group to be different from the side effect profile of the rest of the population, nor that the vaccine can affect unborn babies or breast-fed babies. The side effect reports for this group are still subject to close monitoring.
Further information can be found in a review of new studies completed by the European Medicines Agency (EMA) – read more in the Danish Medicines Agency’s announcement from 20 January 2022 here.
Our vaccine safety monitoring currently also focuses on the following three areas:
Multisystem inflammatory syndrome (MIS)
The Danish Medicines Agency has assessed a single report of a very rare inflammatory condition called MIS-C (Multisystem Inflammatory Syndrome in Children) in a 17-year-old boy. The 17-year-old boy has recovered after treatment. The Danish Medicines Agency has informed the European Medicines Agency (EMA) of the Danish report, and other countries have also observed a few cases of MIS in both children and adults in the time after vaccination.
This led the European Pharmacovigilance Risk Assessment Committee (PRAC) to investigate the possible link between MIS and the four COVID-19 vaccines. See more information in the Danish Medicines Agency’s announcement of 3 September 2021. PRAC has concluded that there is currently insufficient evidence of a possible link between the COVID-19 vaccines and MIS.
See more information in the Danish Medicines Agency’s announcement of 29 October 2021.
The Danish Medicines Agency and the EMA continue their close monitoring of the reports of MIS after COVID-19 vaccination.
Myocarditis and pericarditis
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the membrane around the heart) in the time after vaccination.
Myocarditis and pericarditis are known side effects of Comirnaty (Pfizer/BioNTech) and can in very rare cases occur after vaccination. The symptoms can vary but often include shortness of breath and a forceful heartbeat, that may be irregular, and chest pain.
The Pharmacovigilance Risk Assessment Committee (PRAC) concluded on 3 December 2021 based on the latest safety data that myocarditis and pericarditis can develop within just a few days after vaccination and primarily within 14 days. The current data indicate that the course of myocarditis and pericarditis after vaccination is no different from myocarditis and pericarditis in general.
Menstrual disorders
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of irregular menstrual cycles in the time after vaccination. The reported cases concern heavier periods than usual, delayed periods or period-like bleeding after menopause.
Against this background, the Danish Medicines Agency and the European Medicines Agency (EMA)
have investigated side effect reports involving menstrual disorders and irregular menstrual cycles since the summer 2021.
The reported cases of menstrual disorders have been discussed by the Pharmacovigilance Risk Assessment Committee (PRAC), which has assessed that a causal association between COVID-19 vaccines and menstrual disorders cannot be established so far. See more in the announcement issued by PRAC on 6 August 2021.
Likewise, the Danish Medicines Agency has not been able to establish an association between the Danish reported cases of irregular menstrual cycles and COVID-19 vaccination, but it is an area that is still being monitored closely.
Currently, we have forwarded data from the Danish side effect reports to the European Medicines Agency (EMA) for their further investigations. Find more information in the Danish Medicines Agency’s announcements of 31 August 2021 and 9 December 2021.
News on dkma.dk
Find all of the Danish Medicines Agency’s announcements on pharmacovigilance (safety monitoring of medicines), including COVID-19 vaccines, here. Some announcements are only available in Danish:
/da/nyheder/nyhedskategorier/nyt-om-laegemiddelovervaagning/

Status update on the monitoring of side effects in Denmark
Updated on 18 January 2022.
The Danish Medicines Agency has received 33,252 reports of suspected side effects of Comirnaty (Pfizer/BioNTech), of which we have reviewed 13,688. Reports of serious suspected side effects are reviewed first.
When healthcare professionals, members of the public or their carers/relatives report suspected side effects to the Danish Medicines Agency, only a suspicion is needed to report symptoms they think are side effects of a certain medicine. In other words, a reported suspected side effect does not mean that there is an established connection between the medicine and the experienced symptoms.
The vast majority of suspected side effects reported after vaccination are mild and moderate. They usually involve known and transient side effects such as reactions at the injection site (e.g. pain and itching), tiredness, headache, muscle pain, joint pain, nausea and fever.
When a vaccine activates the immune system, many will experience flu-like symptoms. This is completely normal and a sign that the body’s immune system is reacting to the vaccine. These reactions occur with most vaccine types, and they usually pass within a couple of days.
However, if the immune system does not react, it does not mean that the vaccine is not working. People simply react differently to being vaccinated.
Table 10 below shows the ten most frequently reported suspected side effects (based on cases reviewed).
| The most frequently reported suspected side effects of Comirnaty (Pfizer/BioNTech) as of 18 January 2022 |
|---|
| Headache |
| Fever |
| Tiredness |
| Muscle pain |
| Pain at injection site |
| Nausea |
| Joint pain |
| Dizziness* |
| Chills |
| Feeling unwell |
*Dizziness is not described as a known side effect in the product information, but dizziness may occur in connection with flu-like symptoms, such as fever, chills, tiredness and a feeling of being unwell. These are known side effects of the vaccine.
The table below shows the percentage distribution by age of the reports of suspected side effects received and reviewed by the Danish Medicines Agency for Comirnaty (Pfizer/BioNTech):
| Percentage distribution of reviewed reports by age groups (years) for Comirnaty (Pfizer/BioNTech) as of 18 January 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 | 5-11 | 12-15 | 16-19 | 20-39 | 40-64 | 65-79 | 80+ | Ukendt alder | |
| 0,1% | 0,8% | 2,2 % | 3,2 % | 31,5% | 47,4 % | 8,2 % | 5,5 % | 1,1 % | |
When the Danish Medicines Agency monitors the safety of the COVID-19 vaccines – and medicines in general – we pay particular attention to reports of suspected serious and unexpected side effects. This means that we give first priority to these reports and assess them thoroughly to find out if there is a possible link to the vaccine. In this assessment, we may need to obtain additional information about the side effect.
We also monitor suspected side effects after the COVID-19 booster vaccination. Currently, the Danish Medicines Agency has no reason to suspect that the side effect profile of the booster vaccine is any different from the vaccine’s general side effect profile.
Since the vaccine rollout began in Denmark, we have monitored the below focus areas closely as part of the safety monitoring of the COVID-19 vaccines.
Deaths
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of deaths in the time after vaccination.
The Danish Medicines Agency has so far received and assessed 124 reports involving deaths in the time after vaccination. In all 124 cases, it was assessed to be less likely that the deaths were connected to the vaccine, and that the deaths were more likely to have been caused by other factors.
The reports of deaths are being closely monitored in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Blood clots
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of blood clots in the time after vaccination.
At this time, the Danish Medicines Agency does not suspect the vaccine to cause side effects in the form of blood clots. Blood clots can be explained by several other factors such as the person’s health conditions, including other diseases and medication taken. In addition, the needle prick itself from the vaccine jab may – even when administered correctly – in rare cases cause blood clots. So, blood clots can occur in the time after vaccination without being related to the vaccine.
The reports of blood clots are monitored closely in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Children
The Danish Medicines Agency has particular focus on the monitoring of suspected side effects in the vaccination groups of children aged 5 to 11 years and children aged 12 to 15 years. Based on the available data, the general side effect profile for these age groups is comparable to the observations made for the rest of the population, which primarily involve known and transient side effects.
Pregnancy and breast-feeding
The Danish Medicines Agency has particular focus on the monitoring of suspected side effects in pregnant women and breast-feeding women. The Danish Medicines Agency’s monitoring has given no reason to suspect the side effect profile of this group to be different from the side effect profile of the rest of the population, nor that the vaccine can affect unborn babies or breast-fed babies. The side effect reports for this group are still subject to close monitoring.
Further information can be found in a review of new studies completed by the European Medicines Agency (EMA) – read more in the Danish Medicines Agency’s announcement from 20 January 2022 here.
Our vaccine safety monitoring currently also focuses on the following three areas:
Multisystem inflammatory syndrome (MIS)
The Danish Medicines Agency has assessed a single report of a very rare inflammatory condition called MIS-C (Multisystem Inflammatory Syndrome in Children) in a 17-year-old boy. The 17-year-old boy has recovered after treatment. The Danish Medicines Agency has informed the European Medicines Agency (EMA) of the Danish report, and other countries have also observed a few cases of MIS in both children and adults in the time after vaccination.
This led the European Pharmacovigilance Risk Assessment Committee (PRAC) to investigate the possible link between MIS and the four COVID-19 vaccines. See more information in the Danish Medicines Agency’s announcement of 3 September 2021. PRAC has concluded that there is currently insufficient evidence of a possible link between the COVID-19 vaccines and MIS.
See more information in the Danish Medicines Agency’s announcement of 29 October 2021.
The Danish Medicines Agency and the EMA continue their close monitoring of the reports of MIS after COVID-19 vaccination.
Myocarditis and pericarditis
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the membrane around the heart) in the time after vaccination.
Myocarditis and pericarditis are known side effects of Comirnaty (Pfizer/BioNTech) and can in very rare cases occur after vaccination. The symptoms can vary but often include shortness of breath and a forceful heartbeat, that may be irregular, and chest pain.
The Pharmacovigilance Risk Assessment Committee (PRAC) concluded on 3 December 2021 based on the latest safety data that myocarditis and pericarditis can develop within just a few days after vaccination and primarily within 14 days. The current data indicate that the course of myocarditis and pericarditis after vaccination is no different from myocarditis and pericarditis in general.
Menstrual disorders
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of irregular menstrual cycles in the time after vaccination. The reported cases concern heavier periods than usual, delayed periods or period-like bleeding after menopause.
Against this background, the Danish Medicines Agency and the European Medicines Agency (EMA)
have investigated side effect reports involving menstrual disorders and irregular menstrual cycles since the summer 2021.
The reported cases of menstrual disorders have been discussed by the Pharmacovigilance Risk Assessment Committee (PRAC), which has assessed that a causal association between COVID-19 vaccines and menstrual disorders cannot be established so far. See more in the announcement issued by PRAC on 6 August 2021.
Likewise, the Danish Medicines Agency has not been able to establish an association between the Danish reported cases of irregular menstrual cycles and COVID-19 vaccination, but it is an area that is still being monitored closely.
Currently, we have forwarded data from the Danish side effect reports to the European Medicines Agency (EMA) for their further investigations. Find more information in the Danish Medicines Agency’s announcements of 31 August 2021 and 9 December 2021.
News on dkma.dk
Find all of the Danish Medicines Agency’s announcements on pharmacovigilance (safety monitoring of medicines), including COVID-19 vaccines, here. Some announcements are only available in Danish:
/da/nyheder/nyhedskategorier/nyt-om-laegemiddelovervaagning/
Spikevax (Moderna)
The vaccine’s known side effects appear from the product information of Spikevax (Moderna).
Status update on the monitoring of side effects in Denmark
Updated regulary. Updated on 18 January 2022.
The Danish Medicines Agency has received 8,013 reports of suspected side effects of Spikevax (Moderna), of which we have reviewed 6,489. Reports of serious suspected side effects are reviewed first.
When healthcare professionals, members of the public or their carers/relatives report suspected side effects to the Danish Medicines Agency, only a suspicion is needed to report symptoms they think are side effects of a certain medicine. In other words, a reported suspected side effect does not mean that there is an established connection between the medicine and the experienced symptoms.
The vast majority of suspected side effects reported after vaccination are mild and moderate. They usually involve known and transient side effects such as reactions at the injection site (e.g. pain and itching), tiredness, headache, muscle pain, joint pain, nausea and fever.
When a vaccine activates the immune system, many will experience flu-like symptoms. This is completely normal and a sign that the body’s immune system is reacting to the vaccine. These reactions occur with most vaccine types, and they usually pass within a couple of days.
However, if the immune system does not react, it does not mean that the vaccine is not working. People simply react differently to being vaccinated.
Table 10 below shows the ten most frequently reported suspected side effects (based on cases reviewed).
| The most frequently reported suspected side effects of Spikevax (Moderna) as of 18 January 2022 |
|---|
| Fever |
| Headache |
| Tiredness |
| Pain at injection site |
| Muscle pain |
| Swelling at the injection site |
| Nausea |
| Dizziness |
| Redness at the injection site |
| Joint pain |
The table below shows the percentage distribution by age of the reports of suspected side effects received and reviewed by the Danish Medicines Agency for Spikevax (Moderna).
| Percentage distribution of reports by age groups (years) for Spikevax (Moderna) as of 18 January 2022 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 | 5-11 | 12-15 | 16-19 | 20-39 | 40-64 | 65-79 | 80+ | Ukendt alder | |
| 0,2% | 0,1% | 0,1% | 0,4% | 48,4 % | 32,8 % | 11,3 % | 6,3 % | 0,5 % | |
When the Danish Medicines Agency monitors the safety of the COVID-19 vaccines – and medicines in general – we pay particular attention to reports of suspected serious and unexpected side effects. This means that we give first priority to these reports and assess them thoroughly to find out if there is a possible link to the vaccine. In this assessment, we may need to obtain additional information about the side effect.
We also monitor suspected side effects after the COVID-19 booster vaccination. Currently, the Danish Medicines Agency has no reason to suspect that the side effect profile of the booster vaccine is any different from the vaccine’s general side effect profile.
Since the vaccine rollout began in Denmark, we have monitored the below focus areas closely as part of the safety monitoring of the COVID-19 vaccines.
Deaths
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of deaths in the time after vaccination.
The Danish Medicines Agency has received and assessed 16 reports involving deaths in the time after vaccination. In 15 cases, it was assessed to be less likely that the deaths were connected to the vaccine, and that the deaths were more likely to have been caused by other factors. In one case, it was not possible to assess if the death was caused by the vaccine or the individual’s medical condition.
The reports of deaths are being closely monitored in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Blood Clots
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of blood clots in the time after vaccination.
At this time, the Danish Medicines Agency does not suspect the vaccine to cause side effects in the form of blood clots. Blood clots can be explained by several other factors such as the person’s health conditions, including other diseases and medication taken. In addition, the needle prick itself from the vaccine jab may – even when administered correctly – in rare cases cause blood clots. So, blood clots can occur in the time after vaccination without being related to the vaccine.
The reports of blood clots are monitored closely in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Children
The Danish Medicines Agency has particular focus on the monitoring of suspected side effects in the vaccination groups of children aged 5 to 11 years and children aged 12 to 15 years. Based on the available data, the general side effect profile for these age groups is comparable to the observations made for the rest of the population, which primarily involve known and transient side effects.
Pregnancy and breast-feeding
The Danish Medicines Agency has particular focus on the monitoring of suspected side effects in pregnant women and breast-feeding women. The Danish Medicines Agency’s monitoring has given no reason to suspect the side effect profile of this group to be different from the side effect profile of the rest of the population, nor that the vaccine can affect unborn babies or breast-fed babies. The side effect reports for this group are still subject to close monitoring.
Further information can be found in a review of new studies completed by the European Medicines Agency (EMA) – read more in the Danish Medicines Agency’s announcement from 20 January 2022 here.
Our vaccine safety monitoring currently also focuses on the following two areas:
Myocarditis and pericarditis
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the membrane around the heart) in the time after vaccination.
Myocarditis and pericarditis are known side effects of Spikevax (Moderna) and can in very rare cases occur after vaccination. The symptoms can vary but often include shortness of breath, a forceful heartbeat, that may be irregular, and chest pain.
The Pharmacovigilance Risk Assessment Committee (PRAC) concluded on 3 December 2021 based on the latest safety data that myocarditis and pericarditis can develop within just a few days after vaccination and primarily within 14 days. The current data indicate that the course of myocarditis and pericarditis after vaccination is no different from myocarditis and pericarditis in general.
Menstrual disorders
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of irregular menstrual cycles in the time after vaccination. The reported cases concern heavier periods than usual, delayed periods or period-like bleeding after menopause.
Against this background, the Danish Medicines Agency and the European Medicines Agency (EMA)
have investigated side effect reports involving menstrual disorders and irregular menstrual cycles since the summer 2021.
The reported cases of menstrual disorders have been discussed by the Pharmacovigilance Risk Assessment Committee (PRAC), which has assessed that a causal association between COVID-19 vaccines and menstrual disorders cannot be established so far. See more in the announcement issued by PRAC on 6 August 2021.
Likewise, the Danish Medicines Agency has not been able to establish an association between the Danish reported cases of irregular menstrual cycles and COVID-19 vaccination, but it is an area that is still being monitored closely.
Currently, we have forwarded data from the Danish side effect reports to the European Medicines Agency (EMA) for their further investigations. Find more information in the Danish Medicines Agency’s announcements of 31 August 2021 and 9 December 2021.
News on dkma.dk
Find all of the Danish Medicines Agency’s announcements on pharmacovigilance (safety monitoring of medicines), including COVID-19 vaccines, here. Some announcements are only available in Danish: /da/nyheder/nyhedskategorier/nyt-om-laegemiddelovervaagning/
Vaxzevria (AstraZeneca)
Updated on 18 januar 2022
The vaccine’s known side effects appear from the product information of Vaxzevria (AstraZeneca).
Status update on the monitoring of side effects in Denmark
Updated on 18 January 2022.
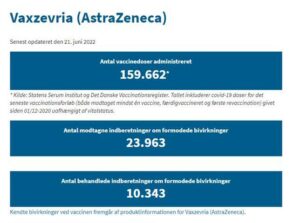
The Danish Medicines Agency has received 23,892 reports of suspected side effects of Vaxzevria (AstraZeneca), of which we have reviewed 6,703. Reports of serious suspected side effects are reviewed first.
When healthcare professionals, members of the public or their carers/relatives report suspected side effects to the Danish Medicines Agency, only a suspicion is needed to report symptoms they think are side effects of a certain medicine. In other words, a reported suspected side effect does not mean that there is an established connection between the medicine and the experienced symptoms.
The vast majority of suspected side effects reported after vaccination are mild and moderate. They usually involve known and transient side effects such as reactions at the injection site (e.g. pain and itching), tiredness, headache, muscle pain, joint pain, nausea and fever.
When a vaccine activates the immune system, many will experience flu-like symptoms. This is completely normal and a sign that the body’s immune system is reacting to the vaccine. These reactions occur with most vaccine types, and they usually pass within a couple of days.
However, if the immune system does not react, it does not mean that the vaccine is not working. People simply react differently to being vaccinated.
Table 10 below shows the ten most frequently reported suspected side effects (based on cases reviewed).
| The most frequently reported suspected side effects of Vaxzevria (AstraZeneca) as of 18 January 2022 |
|---|
| Headache |
| Fever |
| Muscle pain |
| Chills |
| Tiredness |
| Joint pain |
| Pain at injection site |
| Nausea |
| Dizziness |
| Pain |
The table below shows the percentage distribution by age of the reports of suspected side effects received and reviewed by the Danish Medicines Agency for Vaxzevria (AstraZeneca).
| Percentage distribution of reports by age groups (years) for Vaxzevria (AstraZeneca) as of 18 January 2022 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5 | 5-11 | 12-15 | 16-19 | 20-39 | 40-64 | 65-79 | 80+ | Ukendt alder | |||||
| 0% | 0% | 0% | 1% | 40,7 % | 57,2 % | 0,3% | 0% | 0,8% | |||||
When the Danish Medicines Agency monitors the safety of the COVID-19 vaccines – and medicines in general – we pay particular attention to reports of suspected serious and unexpected side effects. This means that we give first priority to these reports and assess them thoroughly to find out if there is a possible link to the vaccine. In this assessment, we may need to obtain additional information about the side effect.
Denmark has not used the Vaxzevria vaccine in the general vaccination programme since 11 March 2021 when the Danish Health Authority put vaccination with Vaxzevria on hold (read the announcement on SST.dk). On 14 April 2021, the Danish Health Authority decided to continue the vaccine rollout without the COVID-19 Vaxzevria vaccine (read the announcement on SST.dk). From 20 May 2021 to 31 August 2021, it was possible for Danes to choose vaccination with Vaxzevria under the optional scheme. The Danish Medicines Agency monitors the vaccine just like all the other vaccines included in the general vaccination programme. Vaxzevria is monitored by drug regulatory authorities around the world like the other COVID-19 vaccines used in Denmark.
Since the vaccine rollout began in Denmark, we have monitored the below focus areas closely as part of the safety monitoring of the COVID-19 vaccines.
Deaths
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of deaths in the time after vaccination.
The Danish Medicines Agency has so far received and assessed four reports involving deaths in the time after vaccination. These two deaths are related to thrombosis with thrombocytopenia syndrome (TTS) as described under the heading below. In addition, the Danish Medicines Agency has received and assessed two reports involving deaths in the time after vaccination. In both cases, it was assessed to be less likely that the deaths were connected to the vaccine, and that the deaths were more likely to have been caused by other factors.
The reports of deaths are being closely monitored in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Reports of blood clots and of unusual symptoms of blood clots in combination with low blood platelets
In Denmark and other countries, reports have been submitted on the very rare, yet serious cases of the combination of blood clots and low platelets, called thrombosis with thrombocytopenia syndrome (TTS) in some cases accompanied by bleeding. In these cases, the blood clots were located at different or unusual sites and with excessive blood coagulation or bleeding throughout the body. Most of these cases occurred within the first three weeks after vaccination. Some cases had a fatal outcome.
The Danish Medicines Agency has reviewed a total of three cases in which it was assessed to be TTS. Two of these three cases involved deaths. The Danish Medicines Agency considers it likely that all three cases could be linked to the vaccine. TTS is described in the product information as a very rare side effect of Vaxzevria.
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of blood clots in the time after vaccination in which there was no combination with low blood platelets (and thus not TTS).
Blood clots can be explained by several other factors such as the person’s health conditions including other diseases and medication taken. In addition, the needle prick itself from the vaccine jab may – even when administered correctly – in rare cases cause blood clots. So, blood clots can occur in the time after vaccination without being related to the vaccine.
Based on safety data, the product information of Vaxzevria was updated to include cerebral venous sinus thrombosis (CVST, blood clots in the brain) without thrombocytopenia (low levels of blood platelets).
The Danish Medicines Agency has received and reviewed very few cases of CVST in the time after vaccination with Vaxzevria.
The reports of deaths are being closely monitored in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
News on dkma.dk
Find all of the Danish Medicines Agency’s announcements on pharmacovigilance (safety monitoring of medicines), including COVID-19 vaccines, here. Some announcements are only available in Danish:
https://laegemiddelstyrelsen.dk/da/nyheder/nyhedskategorier/nyt-om-laegemiddelovervaagning/
Jcovden (Janssen Pharmaceuticals/Johnson & Johnson)

Updated on 18 Januar 2022
Status update on the monitoring of side effects in Denmark
Updated every second week. Updated on 18 January 2022.
The Danish Medicines Agency has received 496 reports of suspected side effects of Jcovden, of which we have reviewed 434. Reports of serious suspected side effects are reviewed first.
When healthcare professionals, members of the public or their carers/relatives report suspected side effects to the Danish Medicines Agency, only a suspicion is needed to report symptoms they think are side effects of a certain medicine. In other words, a reported suspected side effect does not mean that there is an established connection between the medicine and the experienced symptoms.
The vast majority of suspected side effects reported after vaccination are mild and moderate. They usually involve known and transient side effects such as reactions at the injection site (e.g. pain and itching), tiredness, headache, muscle pain, joint pain, nausea and fever.
When a vaccine activates the immune system, many will experience flu-like symptoms. This is completely normal and a sign that the body’s immune system is reacting to the vaccine. These reactions occur with most vaccine types, and they usually pass within a couple of days.
However, if the immune system does not react, it does not mean that the vaccine is not working. People simply react differently to being vaccinated.
Table 10 below shows the ten most frequently reported suspected side effects (based on cases reviewed).
| The most frequently reported suspected side effects of Jcovden (Janssen Pharmaceuticals/Johnson & Johnson) as of 18 January 2022 |
|---|
| Fever |
| Headache |
| Muscle pain |
| tiredness |
| Nausea |
| Joint pain |
| Chills |
| Dizziness |
| Pain at injection site |
| Sensory disturbance in feet, legs, arms or hands |
The table below shows the percentage distribution by age of the reports of suspected side effects received and reviewed by the Danish Medicines Agency for Jcovden (Janssen Pharmaceuticals/Johnson & Johnson).
| Percentage distribution of reports by age groups (years) for Jcovden (Janssen Pharmaceuticals/Johnson & Johnson) as of 18 January 2022 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5 | 5-11 | 12-15 | 16-19 | 20-39 | 40-64 | 65-79 | 80+ | Ukendt alder | |||||
| 0% | 0% | 0% | 0,2% | 89,5% | 8,9% | 0,5% | 0% | 0,9% | |||||
When the Danish Medicines Agency monitors the safety of the COVID-19 vaccines – and medicines in general – we pay particular attention to reports of suspected serious and unexpected side effects. This means that we give first priority to these reports and assess them thoroughly to find out if there is a possible link to the vaccine. In this assessment, we may need to obtain additional information about the side effect.
In Denmark, Jcovden is not currently used in the general vaccination programme.
From 20 May 2021 to 31 August 2021, it was possible for Danes to choose vaccination with Jcovden under the optional scheme. The Danish Medicines Agency monitors the vaccine just like all the other vaccines included in the general vaccination programme. Jcovden is monitored by drug regulatory authorities around the world like the other COVID-19 vaccines used in Denmark.
Since the vaccine rollout began in Denmark, we have monitored the below focus areas closely as part of the safety monitoring of the COVID-19 vaccines.
Deaths
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of deaths in the time after vaccination.
The Danish Medicines Agency has received and assessed one report involving a death in the time after vaccination with COVID-19 Vaccine Janssen. It has been assessed to be less likely that the death was connected to the vaccine, and that the death was more likely to have been caused by other factors.
The reports of deaths are being closely monitored in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
Reports of blood clots and of unusual symptoms of blood clots in combination with low blood platelets
In Denmark and other countries, reports have been submitted on the very rare, yet serious cases of the combination of blood clots and low platelets, called thrombosis with thrombocytopenia syndrome (TTS) in some cases accompanied by bleeding. In these cases, the blood clots were located at different or unusual sites and with excessive blood coagulation or bleeding throughout the body. Most of these cases occurred within the first three weeks after vaccination.
The Danish Medicines Agency has reviewed one case in which it was assessed to be TTS. The Danish Medicines Agency has considered it likely that it could be linked to the vaccine. TTS is described in the product information as a very rare side effect of Jcovden.
Like the drug regulatory authorities in other countries, the Danish Medicines Agency has received reports of blood clots in the time after vaccination in which there was no combination with low blood platelets (and thus not TTS).
Blood clots can be explained by several other factors such as the person’s health conditions including other diseases and medication taken. In addition, the needle prick itself from the vaccine jab may – even when administered correctly – in rare cases cause blood clots. So, blood clots can occur in the time after vaccination without being related to the vaccine.
Venous thromboembolism, a special type of blood clots, is a known, rare side effect of Jcovden.
The reports of blood clots are monitored closely in a collaboration between the Danish Medicines Agency, the European Medicines Agency (EMA) and the other drug regulatory authorities in Europe.
News on dkma.dk
Find all of the Danish Medicines Agency’s announcements on pharmacovigilance (safety monitoring of medicines), including COVID-19 vaccines, here. Some announcements are only available in Danish:
/da/nyheder/nyhedskategorier/nyt-om-laegemiddelovervaagning/
Um höfund

- Sigurlaug Ragnarsdóttir
- ✞༺(((( Ⓒilla ℜągnąℜṧ )))༻♚༺ BA Classical Art Historian || MA Culture & Media || Tourism & Sales Management || Web Design || Photo & Videographer for Tourism Magasins ༻
Síðustu færslur
 PROTECT THE CHILDREN23. nóvember, 2024BARNAMÁLARÁÐSTEFNAN 2024
PROTECT THE CHILDREN23. nóvember, 2024BARNAMÁLARÁÐSTEFNAN 2024 MANNRÉTTINDI19. nóvember, 2024MENNTASPJALL VALGERÐAR SNÆLAND JÓNSDÓTTUR
MANNRÉTTINDI19. nóvember, 2024MENNTASPJALL VALGERÐAR SNÆLAND JÓNSDÓTTUR MANNRÉTTINDI9. ágúst, 2024Lög um borgaralega handtöku voru felld úr gildi árið 2008
MANNRÉTTINDI9. ágúst, 2024Lög um borgaralega handtöku voru felld úr gildi árið 2008 Sameinuðu Þjóðirnar28. desember, 2023Lestrarefni Menntamálastofnunar: Varúð hér býr vampíra – auðlesin sögubók á léttu máli og bókinni fylgir verkefnahefti sem hægt er að vinna samhliða lestri.
Sameinuðu Þjóðirnar28. desember, 2023Lestrarefni Menntamálastofnunar: Varúð hér býr vampíra – auðlesin sögubók á léttu máli og bókinni fylgir verkefnahefti sem hægt er að vinna samhliða lestri.